Kinky molecules
When plants make oils, they prefer kinky molecules. Molecules that are straight can pack together into dense solid clumps, while kinky molecules stay liquid.
What makes the molecules kinky are cis double bonds. Most of the bonds between carbon atoms in fats and oils are single bonds, because most of the carbons are saturated with as many hydrogen atoms as they can hold, and they only have one bond left for the next carbon. Some molecules (monounsaturated fatty acids) have a double bond in them. Some (polyunsaturated fatty acids) have more than one double bond.
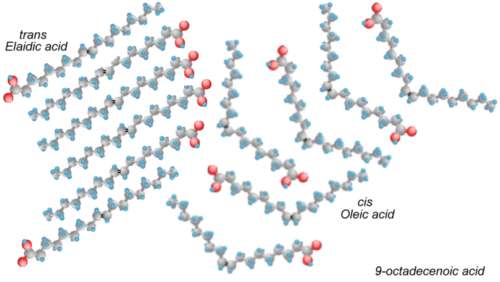
The carbons that share a double bond between them have only one hydrogen each. If the hydrogens are on the same side of the double bond, then the molecule kinks at the bond, forming an angle. If the hydrogens are on opposite sides, then the molecule has no kinks, and it is straight.
The organism that created the fatty acid went to some trouble to make all of the double bonds kinky (called the cis orientation, meaning "on the same side"). If we heat up the oil, we shake up the molecule, and the bonds rearrange somewhat at random, sometimes forming kinky cis bonds, but quite often forming straight trans bonds (meaning "on opposite sides").
Trans fatty acids stack together and form solids. When they are incorporated into the cell walls in arteries, they stiffen the arteries. When they are incorporated into the myelin sheathing of nerve cells in the brain, they can cause neurodegenerative diseases. Trans fats also raise the levels of the bad form of cholesterol (LDL), and lower the levels of the good form (HDL). People who eat trans fats have nearly twice the risk of heart attack as those who avoid them.
Some 20 carbon long cis fatty acids are processed by enzymes to form prostaglandins, prostacyclins, thromboxanes and leucotrienes and other eicosanoids that the body uses to control important processes like the immune system. The trans forms of the same fatty acids don't fit the enzymes, and can't be made into eicosanoids.
Fully hydrogenated fats are also straight molecules, and are generally solids. Since they have no double bonds, they don't oxidize as readily, and so they don't go rancid as quickly as oils. They have longer shelf lives, and form the solid parts of butterfat and lard. To make similar solid fats from vegetable oils, the food industry invented hydrogenation. This is a process where the oils are heated in vessels with compressed hydrogen and some metal catalysts, to add hydrogen to the carbons at the double bonds, converting the bonds to single bonds.
Since fully saturated fats are too solid and wax-like for cooking, the hydrogenation process is not allowed to complete, so the result is a mixture of saturated and unsaturated fats. But because of the high heat used in the process, many of the cis bonds that remain have been converted to trans bonds.
A better process would be to convert all of the unsaturated fatty acids to fully saturated fats, and then to add back some unprocessed oil to thin the mixture to the right consistency. Some trans fat would still remain, since chemical reactions do not usually go fully to completion, but the levels would be much lower. However, it is cheaper to just stop the process when the consistency is right.

3 Comments:
There are some reasonably high-melting cis-unsaturated fatty acids,
http://www.cyberlipid.org/fa/acid0002.htm
petroselinic, vaccenic, gadoleic, erucic (cardiotoxic), nervonic.
Yes, as you get to the very high molecular weights, the melting point rises.
I would expect all of those to be considerably more expensive than hydrogenated fats, but I have not priced them.
Post a Comment
Links to this post:
Create a Link
<< Home